South Dakota Mines Professor Revolutionizes Body's Wound Healing Capabilities
As early as six months after birth, the human body decreases its hyaluronic acid (HA) expression significantly – a vital molecule that supports tissue structure and regulates cell growth and movement.
Found throughout the body, including brain, eyes, joints and skin, HA provides essential lubrication, mechanical support and hydration; however, its decline with age increases the risks of arthritis, cardiovascular issues and impaired wound healing.
While commercial HA products exist, none replicate the body’s natural HA—until now.
Dr. Tugba Ozdemir, South Dakota Mines assistant professor of nanoscience and biomedical
engineering, and her team, which includes undergraduate and graduate students, are
revolutionizing the field with groundbreaking innovations.
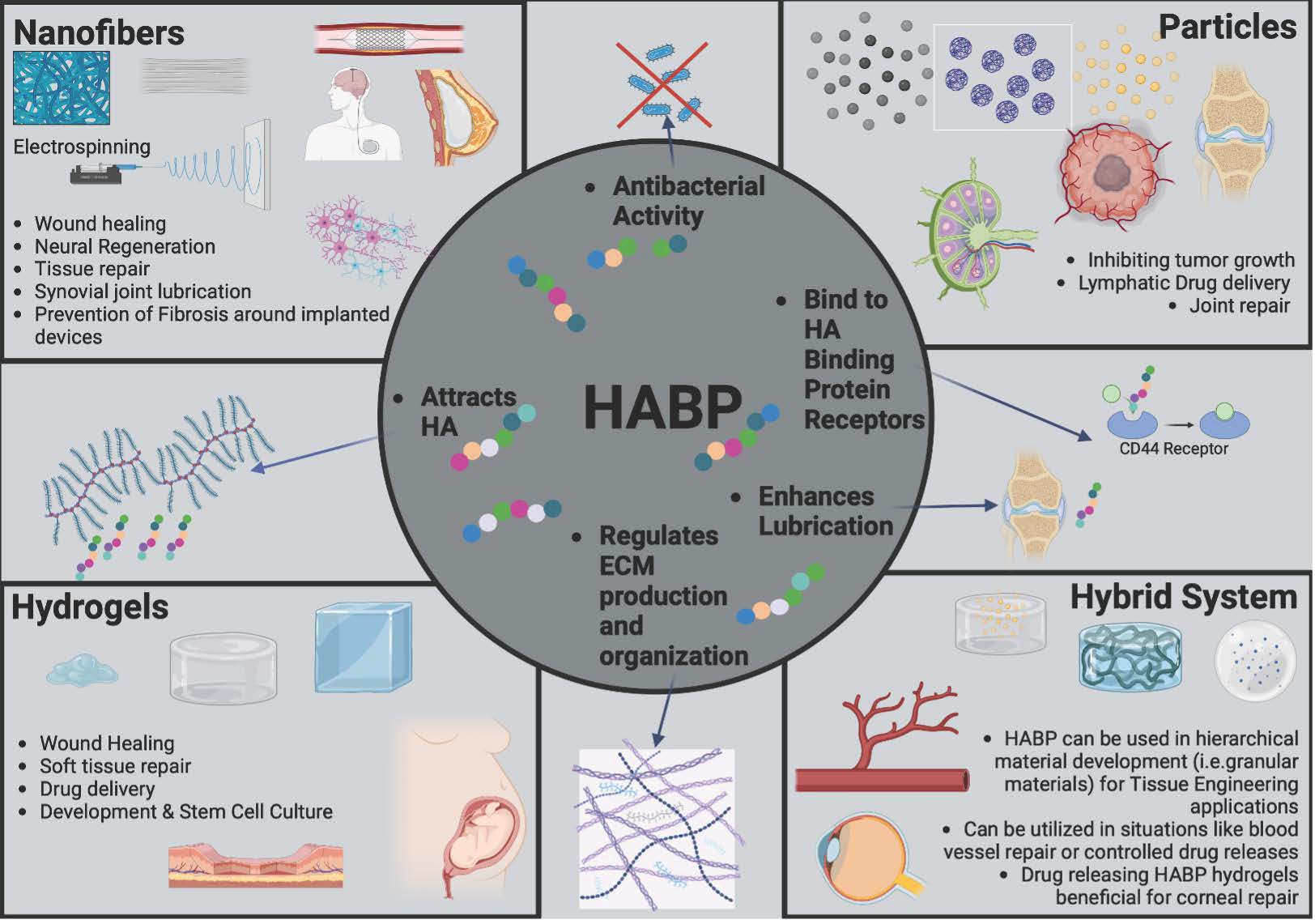
Ozdemir has discovered the use of novel HA-binding peptides (HABPs) which can attract endogenously produced HA to a wound site. “We use these HA binding peptides to attract HA and utilize the body’s ability to produce HA so that endogenous HA can create regenerative responses,” she said.
Ozdemir’s research currently focuses on HABP for wound healing, tissue regeneration and prevention of fibrotic capsule buildup that happens with implants like pacemakers, joint replacements and breast implants. Fibrotic capsule formation is when the body develops a layer of hard, scar-like tissue around a foreign implant. “It is our body’s natural way of protecting itself from this foreign material,” she said.
Following their patent application, Ozdemir’s team participated multiple National Science Foundation’s Innovation Corps (I-Corps) program to work on commercializing their HABP as a coating around the silicone breast implant, intelligent wound dressings and products to reverse skin aging. According to the NSF, the I-Corps program prepares scientists and engineers to extend their focus beyond the university laboratory – accelerating the economic and societal benefits of research projects that are ready to move to commercialization.
“We found that when we coated surfaces with HA-binding peptides, cells produce more HA and the more HA is associated with regenerative healing,” Ozdemir said. “Commercially available HA has been used for wound healing for decades but has been unable to fully prevent fibrosis. Our method utilizes a patient's own, naturally derived HA, which we have discovered has a therapeutic effect on fibroblast cells which are responsible for developing the thick fibrous capsule around the implants.”
With strong funding support from NASA-EPSCOR and Pilot Research award from Drug, Disease and Delivery (3D) Center at South Dakota State University, Ozdemir’s team is developing novel biomaterial systems for wound healing, regeneration and disease modeling.
In Ozdemir’s research, HA-binding biomaterials can overcome the issues of fibrotic capsule formations by combining HABPs with other materials, like collagen, to create dual-function systems. Using advanced technology, synthetic materials can be designed to mimic HA-binding proteins. These biomaterials can take the form of hydrogels, nanofibers or hybrid systems and can be used for applications such as reducing scarring around implants, targeting tumors and treating osteoarthritis.
HA-binding hydrogels hold great potential for repairing soft tissues such as skin, nerves, and the cornea. “Combining hydrogels with particles in hybrid systems could transform treatments for cardiovascular conditions and tissue regeneration,” Ozdemir said. “By harnessing HA’s natural properties, these innovative materials could drive major advancements in healing and regenerative medicine.”